Simultaneous Improvement in Solid-Liquid Separation
and Dissolved Ion Removal from Radioactive Effluents by Polymeric Flocculation
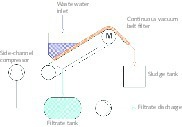
The liquid effluents generated in nuclear industries contain dissolved radioactive Sr and Cs. These species in practices are co-precipitated with synthetically prepared sludge which contains copper ferrocyanide, ferric hydroxide and either calcium phosphate or barium sulphate. Flocculation with some selected polymers enhances the adsorption of the dissolved 90Sr and 137Cs radioactive metal ions from the liquid component of the effluent onto the precipitates by more than two times while using a cationic flocculant. These precipitates have very poor separation properties. The above selected flocculants not only improve the decontamination factor by removing more amounts of the radio active metal ions but also the settling and filtration rates. This paper reports the results and discusses on adsorption studies, settling behaviour and filtration characteristics of the flocculated sludges.
1. Introduction
A wide range of liquid wastes are produced in nuclear industries and in all user industries of radioactive materials. The liquid radioactive wastes generated at nuclear power plants for example, usually contain soluble and insoluble radioactive and non radioactive
components [1]. Such wastes in general need suitable treatment
to reduce the levels of both radioactive as well as non radioactive
contaminants to acceptable limits as per the international conventions and national environmental pollution control regulations before discharging to the environment. Caesium and strontium are...